Platform Technologies
Clade is seeking to dramatically broaden the impact of cell therapy by establishing a more robust cellular platform. To do this, we build cell medicines derived from inherently robust and engineerable induced pluripotent stem cells (iPSCs), and then focus on the core innovations to enable them to work as a “chassis” for subsequent innovations.
Clade’s Platform Technologies are building the foundation for engineerable, off-the-shelf, scalable, and consistent cell medicines:
Cloaking Engine accomplishes broad applicability of our therapies by addressing transplant rejection through our proprietary suite of cloaking edits.
Universal Targeting and enhancements enable our inherently engineerable iPSC-derived cells to outperform autologous cells.
Cell Foundry innovative approach unlocks differentiation of iPSCs into autologous-like cells without the burden of inconsistent, unscalable, and high-cost production processes.
Manufacturing Infrastructure
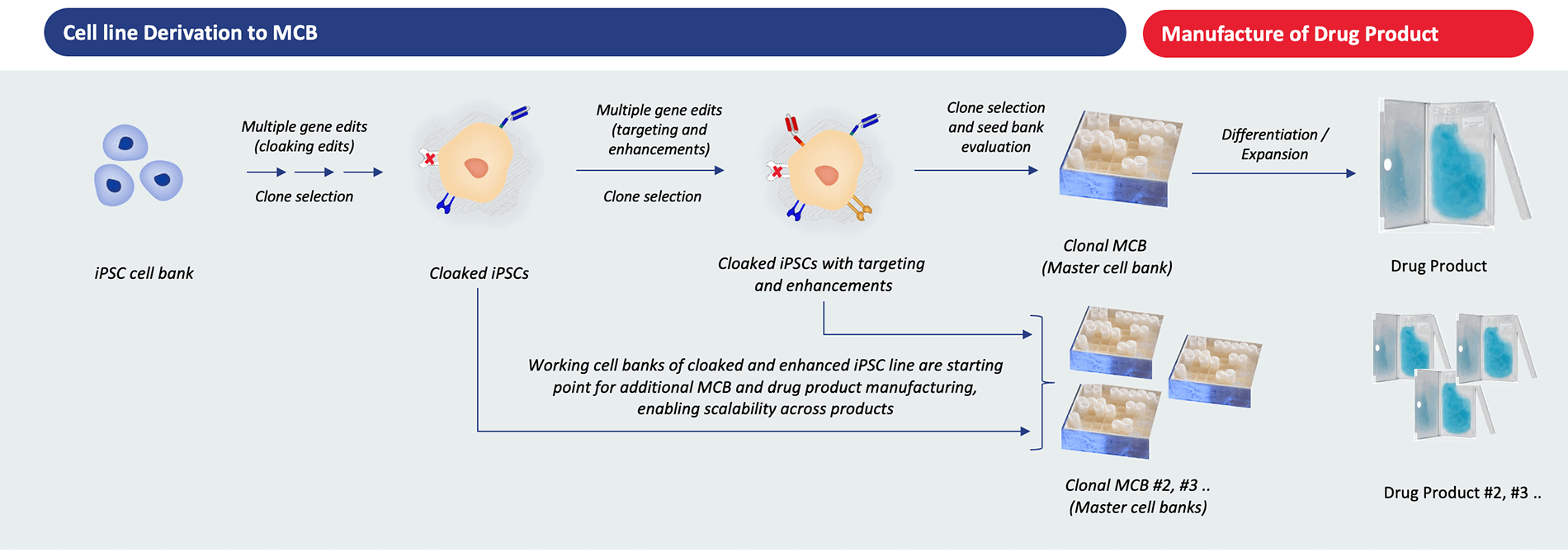
Clade is focused on manufacturing our drug products with a scalable and reproducible process, with consistent quality and precise genomic control.
To enable this, we engineer iPSCs with multiple gene edits that include our cloaking, targeting moieties and cell enhancements, thereby generating a high-quality and well characterized master cell bank (MCB). Finally, we differentiate our engineered iPSCs in a feeder-free, suspension media utilizing our differentiation protocols from our cell foundry to manufacture our drug product.
Clade’s manufacturing process enables significant benefits of scale, consistency, cost, and quality across the manufacturing process.
Pipeline
Clade’s pipeline programs are focused on:
- Replicating the benefits of the autologous CAR-T products, while addressing their profound limitations – inconsistent quality, challenging patient logistics, and lack of scalability.
- Broadening the applicability of cell therapies, e.g., for solid tumor indications, where cell therapies have had underwhelming impact due to the limited engineerability of autologous cells.
Clade’s pipeline is initially focused on harnessing our combination of iPSC derived CD8+ and CD4+ αβ CAR-T cells across multiple autoimmune and solid tumor indications. Clade plans to advance multiple products to the clinic over the next two years.